Introduction: The Growing Importance of Digestive Enzyme Supplements
Digestive enzyme supplements have become increasingly significant in modern health management, partly because digestive discomfort has risen sharply in recent decades and partly because scientific understanding of enzymatic digestion has advanced considerably. In an era where dietary habits are more diverse, processed, and stressful to the gastrointestinal system than ever before, many individuals experience a functional gap between what they eat and what their digestive capacity can effectively handle. As a result, digestive enzymes—once considered niche aids reserved for extreme insufficiencies—are now recognized as essential tools that help optimize digestion, support nutrient absorption, and reduce the burden on an already overtaxed gastrointestinal system.
Human digestion depends on a precisely orchestrated series of biochemical reactions in which enzymes break down complex nutrients into absorbable units. When the body’s endogenous enzymes are insufficient, digestion becomes inefficient, leading to a variety of consequences such as bloating, abdominal discomfort, nutrient malabsorption, and shifts in microbiome composition. These issues are not limited to clinical diseases; they are also common among healthy individuals who simply consume meals that exceed their enzymatic capacity, eat too quickly, or regularly follow high-fat, high-protein, or fiber-heavy diets.
There are several reasons why enzyme insufficiency has become more common. Aging, for example, naturally reduces the output of stomach acid and pancreatic enzymes, thereby slowing the early stages of protein and fat digestion. Chronic stress exerts a direct inhibitory effect on digestive secretions by altering autonomic balance, reducing vagal tone, and suppressing salivary flow and gastric output. The widespread use of proton-pump inhibitors, antacids, and other medications further interferes with enzyme activation pathways. Added to this are inflammatory conditions—such as IBS, IBD, SIBO, and post-infectious gut dysfunction—that damage the intestinal brush border, the very surface where the body’s final-stage digestive enzymes reside. Consequently, a significant portion of the population today experiences digestive symptoms that stem not from intolerance per se, but from impaired enzymatic activity.
Digestive enzyme supplements offer a direct and scientifically grounded solution to these challenges. By providing exogenous enzymes that complement or replace endogenous function, these supplements help ensure that carbohydrates, fats, proteins, and fibers are broken down efficiently. This leads not only to improved digestive comfort but also enhanced nutrient availability, better metabolic balance, and reduced gastrointestinal stress. Clinical research consistently demonstrates that digestive enzymes can significantly improve symptoms in conditions such as lactose intolerance, IBS, functional dyspepsia, and exocrine pancreatic insufficiency, as well as support individuals who have undergone gallbladder removal or bariatric surgery.
The growing demand for digestive enzyme supplements has also been supported by major advancements in formulation science. Modern enzyme supplements are no longer limited to basic pancreatin or lactase preparations. They now include sophisticated multi-enzyme complexes derived from plant, fungal, and microbial sources, offering broad-spectrum activity across varying pH ranges and meal compositions. Such formulations require strict manufacturing controls to maintain enzyme stability, potency, and activity across the product’s shelf life, making digestive enzymes one of the most technically challenging product categories in the nutraceutical industry.
Understanding digestive enzyme supplements requires a deep foundation in digestive physiology. Before discussing the types of enzyme supplements and their clinical applications, it is essential to examine how the body’s digestive system works, how enzymes function at each stage of digestion, and what happens when these enzymes are insufficient. These insights create the scientific framework for appreciating the value and mechanisms of digestive enzyme supplementation in modern health.
Human Digestive Physiology and the Central Role of Enzymes
Human digestion is a remarkably coordinated biochemical system that relies on precise enzymatic activity from the moment food enters the mouth until nutrients are absorbed in the small intestine. Each region of the digestive tract provides distinct conditions—ranging from highly acidic to neutral or slightly alkaline environments—that enable specific enzymes to function optimally. Understanding this physiology is essential for appreciating why digestive enzyme insufficiency leads to discomfort, malabsorption, and systemic nutrient deficiencies, and why supplemental enzymes can so effectively restore digestive balance.
Digestion begins in the oral cavity, where food is mechanically broken down through mastication and simultaneously exposed to salivary enzymes. Salivary amylase, also known as ptyalin, initiates the breakdown of complex carbohydrates into smaller dextrins and maltose, setting the stage for more advanced starch digestion later in the gastrointestinal tract. Although often overlooked, the oral phase plays a significant metabolic role. Individuals who chew poorly or eat rapidly limit the action of salivary amylase, leaving larger carbohydrate fragments to burden downstream digestion. Stress and dehydration further impair salivary enzyme output, diminishing early-phase carbohydrate hydrolysis. Lingual lipase, a lesser-known enzyme secreted by glands at the back of the tongue, begins the digestion of dietary fats. While more active in infants, lingual lipase continues to contribute to fat digestion in adults and prepares lipids for subsequent enzymatic action in the stomach and small intestine.
Once food enters the stomach, digestion enters a dramatically different biochemical environment characterized by extremely low pH. Hydrochloric acid unfolds complex proteins, making them accessible to enzymatic cleavage. This acidic environment is also crucial for converting inactive pepsinogen into pepsin, one of the body’s most powerful proteases. Pepsin breaks long-chain proteins into shorter peptides, a process essential for efficient digestion of meats, dairy proteins, legumes, and other protein-dense foods. Unfortunately, the production of stomach acid decreases naturally with age and is further suppressed by medications such as proton-pump inhibitors (PPIs). As a result, the stomach may fail to activate adequate amounts of pepsin, leaving proteins insufficiently digested and contributing to sensations of heaviness, fullness, or bloating.
Gastric digestion also relies on gastric lipase, an enzyme responsible for approximately 10–30% of total fat digestion. Although pancreatic lipase performs the majority of fat hydrolysis, gastric lipase initiates this process under acidic conditions and becomes particularly important when pancreatic function is impaired or when meals contain large amounts of fat. In addition to its enzymatic activities, the stomach churns food and mixes it into chyme, a semi-liquid substance that optimizes the exposure of nutrients to digestive enzymes further down the tract.
As chyme enters the duodenum, the body orchestrates a shift from acidic to alkaline conditions to prepare for the activity of pancreatic enzymes. The pancreas releases a concentrated mixture of amylase, lipase, trypsin, chymotrypsin, and other proteases, which collectively complete the majority of enzymatic digestion. Pancreatic amylase continues the breakdown of starches into simple sugars, while trypsin and chymotrypsin degrade peptides into even smaller fragments. Pancreatic lipase—supported by its cofactor colipase—is the key enzyme in fat digestion, hydrolyzing triglycerides into monoglycerides and free fatty acids. Together, these breakdown products can be absorbed through the intestinal mucosa, provided that sufficient bile salts are present to emulsify dietary fats. The pancreas also secretes nucleases, enzymes that digest nucleic acids into nucleotides, contributing to the absorption of genetic material derived from food.
The small intestine itself plays a sophisticated metabolic role through its brush-border enzymes, located on the microvilli lining the intestinal wall. These enzymes finish the final stage of digestion and produce the actual absorbable molecules that enter circulation. Lactase breaks lactose into glucose and galactose; sucrase-isomaltase converts sucrose and dextrins into simple sugars; maltase and glucoamylase complete the digestion of starch-derived disaccharides; and aminopeptidases reduce small peptides into amino acids ready for absorption. Among the most clinically relevant enzymes is alpha-galactosidase, which breaks down complex carbohydrates found in beans, legumes, and cruciferous vegetables. When this enzyme is insufficient, these carbohydrates reach the colon intact, where they undergo fermentation by gut bacteria, leading to gas, bloating, and abdominal pressure.
What makes the small intestine particularly vulnerable is its sensitivity to inflammation. Even mild inflammatory changes—common in conditions like IBS, SIBO, celiac disease, or post-infectious gut dysfunction—can impair brush-border enzyme expression. When microvilli become blunted or inflamed, lactase and sucrase levels drop significantly, leading to food intolerances that may only appear later in life. The resulting maldigestion not only produces symptoms but also alters the microbiome by providing fermentable substrates to bacteria in regions where fermentation should not normally occur.
Digestive physiology depends on a finely tuned collaboration between mechanical processing, hormonal signaling, pH modulation, and enzymatic catalysis. Any disruption in one phase places added stress on the next, meaning that enzyme insufficiency—whether in the stomach, pancreas, or small intestine—can quickly cascade into systemic digestive disturbances. These physiological realities underpin the growing reliance on digestive enzyme supplements as external support for a system that is frequently strained by modern diets, lifestyle patterns, and age-related changes.
Causes of Digestive Enzyme Insufficiency
Digestive enzyme insufficiency arises from a wide spectrum of biological, environmental, and lifestyle-related factors that collectively compromise the body’s ability to break down food efficiently. Although severe insufficiency is traditionally associated with pancreatic disease, modern clinical evidence shows that even mild disruptions occurring anywhere along the digestive tract can meaningfully impair enzymatic performance. Understanding the causes of enzyme insufficiency requires examining not only pathological processes but also the more subtle physiological and environmental influences that shape the digestive environment.
One of the most significant contributors to declining digestive enzyme activity is the natural aging process. As individuals grow older, several aspects of digestion change simultaneously. The stomach produces less hydrochloric acid, reducing the activation of pepsin, the primary protease responsible for initiating protein breakdown. Lower gastric acidity also weakens the chemical signal that normally triggers robust pancreatic enzyme secretion when chyme enters the small intestine. In addition, the exocrine function of the pancreas gradually diminishes with age, meaning that older adults often secrete fewer digestive enzymes than younger individuals, even if they appear otherwise healthy. This age-related decline contributes to the common experience of post-meal heaviness or intolerance to high-fat or high-protein foods in middle-aged and elderly populations.
Dietary patterns strongly influence enzyme sufficiency as well. Modern diets frequently contain large quantities of saturated fats, processed carbohydrates, refined sugars, and ultra-processed foods that require extensive enzymatic effort to digest. High-fat meals, for example, place heavy demands on pancreatic lipase and bile acids, and individuals who habitually consume such meals often exceed their natural lipase capacity, resulting in steatorrhea or gas from undigested fats. Similarly, high-protein diets require substantial protease output; when the pancreas cannot keep pace with this demand, proteins remain partially digested, leading to fermentation, increased gas production, and digestive discomfort. Ultra-processed foods, which typically contain artificial stabilizers, emulsifiers, and other additives, may impair gastric motility and alter the gastrointestinal environment in ways that make enzymatic digestion more difficult.
Chronic psychological stress is another underappreciated factor contributing to enzyme insufficiency. The digestive system is tightly regulated by the autonomic nervous system, particularly the parasympathetic (rest-and-digest) branch. Under conditions of chronic stress, sympathetic activation reduces salivary output, slows gastric acid secretion, and blunts pancreatic enzyme release. These changes compromise digestion at the earliest stages, producing a cascade of maldigestion that may manifest as bloating, reflux, cramping, or food intolerances. Stress also delays gastric emptying and alters intestinal motility, further upsetting the timing and environment in which enzymes must function.
Inflammation within the gastrointestinal tract has perhaps the most direct impact on enzyme activity, especially at the level of the brush border. The microvilli that line the small intestine host lactase, sucrase, maltase, glucoamylase, peptidases, and numerous other enzymes responsible for the final steps of digestion. When the intestinal mucosa is inflamed—whether due to infection, IBS, IBD, SIBO, or non-celiac gluten sensitivity—these microvilli can become blunted or dysfunctional. Even mild inflammation, invisible in routine medical tests, can significantly reduce brush-border enzyme expression. As a result, lactose intolerance, sucrose intolerance, or even generalized carbohydrate malabsorption may appear unexpectedly in individuals who previously tolerated these foods without issue.
Medication use also plays a major role. Proton-pump inhibitors, designed to reduce stomach acid, are among the most commonly prescribed drugs worldwide. While highly effective for reflux, they unintentionally impair the activation of pepsin and disrupt the acid-to-alkaline signaling cascade necessary for proper pancreatic function. Antibiotics alter microbiome composition, which has downstream effects on mucosal integrity and brush-border enzyme synthesis. Diabetes medications such as metformin influence intestinal motility and can provoke digestive symptoms that stem from subtle enzyme insufficiency rather than intrinsic intolerance. Even pain medications that slow gastrointestinal transit may lead to fermentation and symptoms that resemble enzyme deficiency.
Beyond these physiological and pharmacological influences, genetic factors also contribute to digestive enzyme insufficiency. Lactase non-persistence—a condition in which lactase activity declines naturally after childhood—is the default genetic pattern for most of the world’s population. People with congenital sucrase-isomaltase deficiency lack full ability to break down sucrose or starch-derived polysaccharides, leading to chronic diarrhea, gas, and abdominal discomfort that are frequently misdiagnosed as IBS. Less common genetic conditions affecting pancreatic enzyme synthesis or enzyme activation can also produce profound digestive impairment.
A more severe but clinically important category of enzyme insufficiency arises from pancreatic disorders. Chronic pancreatitis, pancreatic cancer, cystic fibrosis, alcohol-induced pancreatic injury, and postoperative changes following pancreatic or gastrointestinal surgery all reduce exocrine pancreatic output. These individuals may experience dramatic malabsorption of fats, proteins, and fat-soluble vitamins. Even in milder cases, pancreatic insufficiency creates a bottleneck in the digestive process that supplemental enzymes can help alleviate.
Lifestyle and environmental factors compound many of these issues. Rapid eating restricts the time for both mechanical and enzymatic digestion in the mouth. Dehydration reduces salivary enzyme output and diminishes mucosal hydration in the intestines. Smoking impairs pancreatic function and acid production, while excessive alcohol intake may damage pancreatic cells and disrupt enzyme secretion. Together, these influences highlight that enzyme insufficiency is not solely a medical diagnosis but a broader physiological phenomenon shaped by daily habits, dietary choices, stress, and aging.
Understanding the multitude of factors that can diminish enzymatic activity is fundamental to recognizing why digestive enzymes have become so useful in modern health. Whether the insufficiency originates from inflammation, aging, stress, pancreatic dysfunction, or dietary imbalance, the end result is the same: nutrients remain partially digested, digestive organs become overburdened, and symptoms arise. Supplemental digestive enzymes offer a practical and effective way to compensate for these deficits, restoring digestive efficiency and reducing the discomfort associated with modern eating patterns.
Types of Digestive Enzyme Supplements
Digestive enzyme supplements encompass a diverse range of biologically active compounds sourced from animals, plants, and fungi, each offering distinct catalytic properties and digestive applications. Although often marketed as simple aids for general digestion, these enzymes differ profoundly in their biochemical behavior, pH tolerance, substrate specificity, and suitability for different dietary patterns or physiological conditions. Understanding these distinctions is crucial both for clinicians who recommend enzyme therapy and for manufacturers who formulate high-performance blends.
Animal-derived enzymes, most notably pancreatin, represent the closest analogue to the body’s own enzymatic secretions. Pancreatin is typically extracted from porcine or bovine pancreas and contains a mixture of proteases, amylase, and lipase that collectively mirror the exocrine output of the human pancreas. These enzymes function optimally in the alkaline conditions of the small intestine and are capable of breaking down nearly all major nutrient categories, including proteins, fats, and carbohydrates. Pancreatin has long been used in prescription therapies for exocrine pancreatic insufficiency, where it dramatically improves fat and protein absorption and reduces symptoms such as steatorrhea, weight loss, and malnutrition. However, because animal-derived enzymes are sensitive to acidic environments, they commonly require enteric coating to protect their catalytic activity as they pass through the stomach. Additionally, pancreatin is unsuitable for vegan consumers and must be carefully sourced for compliance with Halal and Kosher standards.
Alongside pancreatin, ox bile extract plays a specialized but important role in fat digestion. Although not an enzyme itself, bile assists lipase by emulsifying dietary fats into micelles, thereby increasing their surface area and allowing pancreatic lipase to act more effectively. Individuals who have undergone gallbladder removal, or who experience impaired bile flow, often find that enzyme supplements containing ox bile significantly reduce nausea, bloating, and fatty stool after high-fat meals. When combined with lipase, ox bile can mimic the physiological conditions required for efficient fat hydrolysis, making such combinations highly effective for individuals with fat intolerance.
Plant-derived enzymes form another major category and are particularly valued for their broad pH stability and unique proteolytic capabilities. Bromelain, extracted from the stem of pineapple, is a cysteine protease known for its ability to degrade a wide range of protein substrates across both acidic and neutral environments. This versatility makes bromelain a valuable addition to digestive formulations aimed at heavy or protein-rich meals, such as those containing meat or dairy proteins. Papain, derived from papaya, offers similar proteolytic activity but with a gentler profile, making it suitable for individuals with more sensitive digestion. Actinidin, an enzyme naturally found in kiwi fruit, possesses remarkable ability to break down casein, whey proteins, and certain plant proteins that are notoriously difficult for some individuals to digest. Likewise, enzymes extracted from ginger, such as zingibain, support protein degradation and may aid in improving gastric motility in people with delayed stomach emptying.
Fungal-derived enzymes, primarily produced from species such as Aspergillus oryzae and Aspergillus niger, represent the most versatile and widely used category in modern enzyme supplementation. These microbial enzymes offer broad-spectrum activity and function effectively across a wide pH range, making them ideal for multi-enzyme complexes intended for general digestive support. Fungal amylase is particularly effective in breaking down starches into maltose and glucose, while fungal lipase demonstrates superior stability in varying pH environments compared to its pancreatic counterpart. Cellulase, also derived from fungal fermentation, plays a unique role because humans do not naturally produce cellulase. This enzyme helps break down insoluble plant fibers and reduces the gas and bloating that often accompany diets high in vegetables, legumes, and whole grains. Other enzymes, such as xylanase, hemicellulase, and pectinase, target the polysaccharides commonly associated with fermentable carbohydrates (FODMAPs) and are valuable for individuals who suffer from gas and bloating triggered by plant-rich meals.
Lactase, another well-studied fungal enzyme, holds a special place in digestive supplementation due to its overwhelming clinical evidence in lactose intolerance. Individuals lacking endogenous lactase experience significant symptoms when consuming dairy products, and supplementing with exogenous lactase before meals reliably improves tolerance by breaking lactose into absorbable monosaccharides. Similarly, alpha-galactosidase, an enzyme that breaks down complex oligosaccharides found in beans and cruciferous vegetables, has been shown to reduce gas production and abdominal distension in individuals sensitive to these foods. These enzymes are particularly useful for people who experience discomfort after consuming high-fiber, plant-based meals or for individuals with mild carbohydrate malabsorption.
Beyond these core categories, specialized enzyme blends have emerged to target specific digestive challenges. Anti-FODMAP formulations combine alpha-galactosidase, cellulase, xylanase, and related enzymes to reduce the fermentable substrates that cause bloating in individuals with IBS or functional bloating. Dairy digestion blends combine lactase with proteases and lipase to aid not only in lactose breakdown but also in the digestion of casein and milk fats. Gluten-specific enzyme formulations are designed to hydrolyze gluten peptides; while they cannot replace a gluten-free diet in individuals with celiac disease, they may help reduce symptoms associated with accidental gluten exposure in those with non-celiac gluten sensitivity.
Lipase-focused formulations provide heightened support for individuals who struggle primarily with fat digestion, including people following ketogenic or high-fat diets and those who have difficulty digesting fatty foods due to compromised bile flow. These specialized blends often incorporate bile salts, phospholipases, and enhanced lipase concentrations to mimic the physiological mechanisms involved in fat emulsification and breakdown.
What distinguishes advanced digestive enzyme formulations from simple single-enzyme products is the deliberate synergy between enzymes with complementary functions. Multi-enzyme complexes are designed to work across the varying pH landscapes of the digestive tract, with certain enzymes performing optimally in the acidic stomach and others activating in the neutral to alkaline environment of the small intestine. Such formulations account for differences in substrate types—proteins, fats, starches, disaccharides, or fibers—and are crafted to emulate the multi-layered enzymatic landscape of physiological digestion.
The diversity of digestive enzyme supplements reflects the complexity of human digestion itself. Each enzyme fulfills a particular role, and understanding these roles allows for precise formulation that can address the broad range of digestive challenges encountered in modern dietary patterns. By selecting enzymes with the appropriate specificity, stability, and pH range, formulators can create products that provide meaningful improvements to digestive comfort and nutrient absorption in targeted populations.
Clinical Evidence and Applications of Digestive Enzyme Supplementation
The clinical relevance of digestive enzyme supplementation has expanded significantly in recent years, supported by a growing body of scientific research demonstrating its effectiveness across a wide range of gastrointestinal and metabolic disturbances. While enzyme therapy is traditionally associated with pancreatic insufficiency, modern studies show that supplemental enzymes can meaningfully improve digestive outcomes in conditions such as irritable bowel syndrome (IBS), functional dyspepsia, lactose intolerance, small intestinal bacterial overgrowth (SIBO), post-cholecystectomy fat intolerance, and even general post-meal discomfort in otherwise healthy individuals. The underlying mechanisms vary depending on the enzyme and condition, but they share a common premise: when endogenous enzyme output is inadequate, digestion becomes incomplete, symptoms arise, and supplementation restores the biochemical steps necessary for comfortable digestion and efficient nutrient absorption.
Irritable bowel syndrome has been one of the most extensively studied conditions in relation to enzyme supplementation. Although IBS does not involve structural damage to the gut, its functional disturbances frequently include compromised carbohydrate digestion and heightened sensitivity to gas produced by microbial fermentation. Research reveals that many IBS patients exhibit reduced activity of brush-border enzymes—particularly lactase, sucrase, and maltase—which results in undigested sugars reaching the colon, where they undergo rapid fermentation. This process contributes to bloating, distension, cramping, and altered bowel movements. Clinical trials show that enzymes targeting these fermentable carbohydrates, especially alpha-galactosidase and lactase, help reduce symptom severity by breaking down substrates before they reach gas-producing microbes. Individuals with IBS-M (mixed type) and IBS-D (diarrhea-predominant) appear to benefit the most, supported by both subjective symptom improvement and objective reductions in breath hydrogen levels.
Functional dyspepsia, another highly prevalent condition characterized by early satiety, post-meal fullness, nausea, and discomfort in the upper abdomen, also shows strong response to digestive enzyme therapy. In these individuals, gastric motility is often impaired and the stomach may struggle to efficiently process proteins and fats, creating a sensation of heaviness after meals. Studies using multi-enzyme supplements containing proteases and lipase demonstrate improvements in post-prandial comfort, suggesting that reducing the biochemical workload on the stomach allows food to empty more efficiently. Some research also indicates that proteolytic enzymes such as bromelain may exert mild anti-inflammatory effects on the gastric mucosa, further supporting their use in patients who report pressure or discomfort shortly after eating.
Among all digestive disorders, lactose intolerance provides the most robust clinical evidence for enzyme supplementation. Lactase enzyme therapy has been validated through decades of research and is widely recognized as one of the most effective non-pharmaceutical interventions for a food intolerance. Individuals who lack sufficient endogenous lactase experience rapid fermentation of lactose in the colon, leading to gas, diarrhea, and abdominal pain. Supplementing with exogenous lactase prior to consuming dairy reliably prevents these symptoms by hydrolyzing lactose into glucose and galactose before it reaches the colon. Numerous randomized, placebo-controlled trials have confirmed its efficacy, and breath hydrogen tests consistently show reduced fermentation when lactase is administered.
Small intestinal bacterial overgrowth presents a different but related digestive challenge. SIBO occurs when excessive bacteria colonize the small intestine, consuming nutrients prematurely and producing gas in a region of the gut that is not designed for fermentation. Although enzymes do not directly treat SIBO, they play a supportive role by accelerating digestion and reducing the amount of partially digested carbohydrates available to pathogenic bacteria. Individuals with SIBO often experience immediate post-meal bloating because bacteria ferment nutrients before the body can digest them. Enzymes such as lactase, alpha-galactosidase, and xylanase can reduce symptom intensity by decreasing the substrate load available for bacterial metabolism. This approach is especially helpful when combined with antimicrobial or dietary therapies commonly used in SIBO management.
Exocrine pancreatic insufficiency (EPI) remains the clinical condition for which enzyme therapy is not only beneficial but essential. In EPI, the pancreas fails to secrete adequate amounts of lipase, amylase, and proteases, resulting in profound malabsorption of fats, proteins, and fat-soluble vitamins. Patients commonly experience weight loss, oily stools, abdominal cramping, and nutritional deficiencies. Prescription-strength pancreatic enzyme replacement therapy (PERT) remains the gold standard of care, but individuals with milder forms of pancreatic dysfunction often benefit from high-potency over-the-counter lipase- and protease-rich formulations. Studies show significant improvements in stool quality, nutrient absorption, and symptom relief when appropriate doses of enzymes are administered with meals.
Another population that responds remarkably well to digestive enzymes consists of individuals who have undergone gallbladder removal. Without a gallbladder to regulate bile release, bile trickles continuously into the small intestine rather than being available in sufficient quantities during meals. This results in suboptimal emulsification of fats and leads to symptoms such as bloating, nausea, and fatty stools. Enzyme supplements containing enhanced lipase activity and, in some cases, ox bile extract, help recreate the physiological conditions needed for effective lipid digestion. Clinical outcomes indicate reductions in post-meal discomfort and improvements in tolerance to fat-rich foods.
People following high-fat or high-protein diets—such as ketogenic or bodybuilding regimens—also frequently report improvements when using digestive enzymes. High-protein diets demand substantial protease activity, and when this demand exceeds endogenous production, undigested protein can lead to fermentation and discomfort. Similarly, high-fat meals place a heavy burden on lipase and bile production. Supplemental enzymes ease these mechanical and biochemical burdens, allowing individuals to maintain their dietary preferences without experiencing gastrointestinal distress.
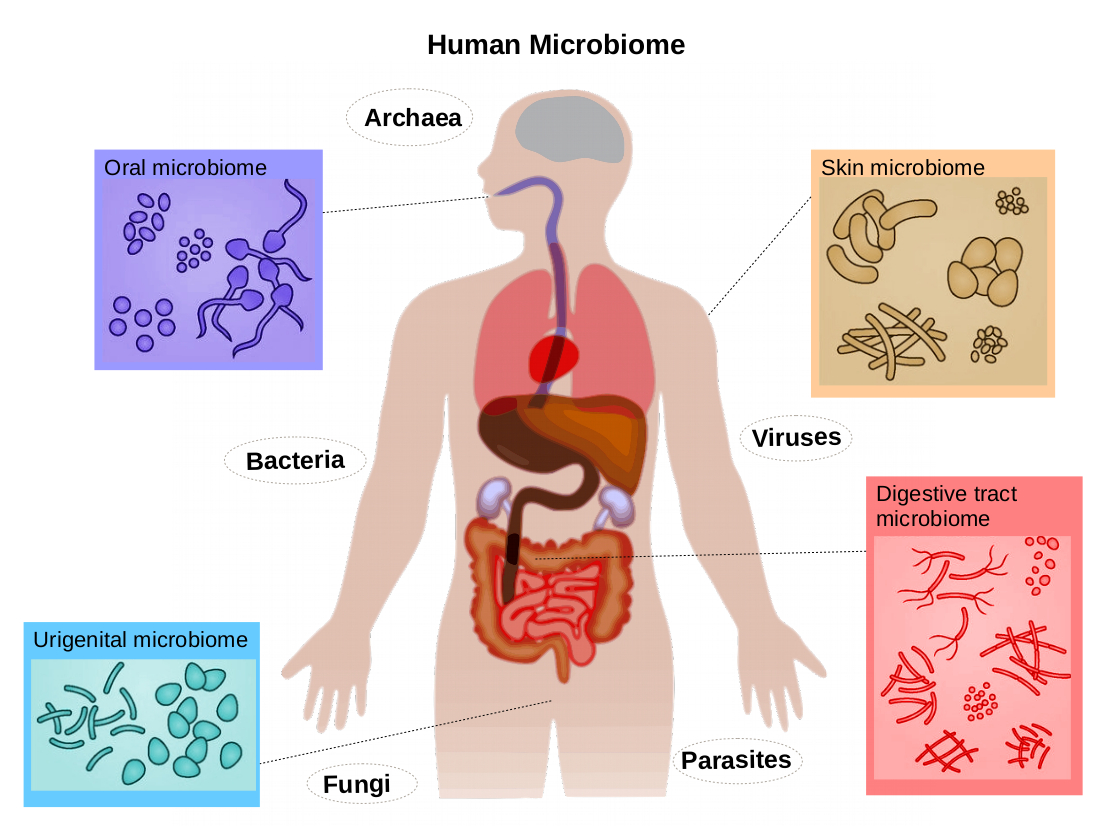
Enzymes also influence the gut microbiome in subtle but important ways. By improving the breakdown and absorption of nutrients in the small intestine, they reduce the volume of undigested material that reaches the colon. This prevents excessive fermentation, which in turn reduces gas, bloating, and pressure. More importantly, it shifts the microbial environment toward a healthier balance by reducing substrate availability for opportunistic bacteria. Studies examining combinations of digestive enzymes and probiotics show that enzymes can enhance the survival of beneficial bacteria by improving the biochemical environment of the gut.
Another clinically significant but less frequently discussed aspect of enzyme therapy is its effect on nutrient absorption. A substantial portion of individuals with chronic digestive symptoms also display deficiencies in essential nutrients—including iron, magnesium, vitamin B12, amino acids, and fat-soluble vitamins—not because these nutrients are lacking in their diet, but because inefficient digestion prevents proper absorption. By restoring complete hydrolysis of proteins, fats, and carbohydrates, digestive enzymes indirectly improve the availability of these nutrients for absorption across the intestinal mucosa.
Taken together, the clinical evidence clearly positions digestive enzymes as versatile therapeutic tools capable of improving a wide range of digestive challenges. Whether the insufficiency originates from carbohydrate malabsorption, protein intolerance, fat digestion difficulties, microbial imbalance, pancreatic dysfunction, or structural changes following surgery, enzyme supplementation provides a direct and scientifically supported way to restore the biochemical steps necessary for comfortable and efficient digestion. The breadth of evidence across diverse populations underscores the central role that enzymatic function plays in digestive health and highlights why supplemental enzymes have become an indispensable component of modern gastrointestinal care.
Safety, Contraindications, and Dosage Considerations for Digestive Enzymes
Digestive enzyme supplements are generally recognized as safe and well tolerated, owing to their long history of use in both clinical and nutritional settings. Nevertheless, because enzymes are biologically active proteins with specific catalytic functions, their safety and effectiveness depend on appropriate dosing, correct timing, and awareness of underlying medical conditions that may modify their impact. A nuanced understanding of these factors is essential for clinicians, formulators, and consumers seeking to use digestive enzymes responsibly and effectively.
In most individuals, digestive enzymes produce few side effects when taken with meals, which is the condition under which they are designed to function. When used correctly, enzymes do not enter systemic circulation in meaningful quantities during digestion; instead, they act locally within the gastrointestinal lumen, breaking down proteins, carbohydrates, fats, and fibers into smaller, absorbable molecules. Because their activity is confined to the digestive tract, adverse events tend to be mild and transient, often limited to sensations of increased digestive activity such as warmth in the stomach or temporary shifts in stool consistency as digestion becomes more efficient. These responses typically subside as the body adjusts.
Despite this strong safety profile, certain conditions warrant caution. Individuals with active gastritis or peptic ulcers may find that high-potency proteases aggravate their symptoms. Proteolytic enzymes, by design, hydrolyze proteins, and although they primarily target dietary proteins, they can irritate compromised gastric tissue when acid barriers are weakened. In such cases, gentler formulations or reduced dosages may be preferable. Similarly, people with known allergies to pineapple, papaya, or other enzyme-source plants should avoid bromelain or papain, as sensitivities can occasionally extend to the enzymes themselves. Animal-derived enzymes like pancreatin may also present allergenic concerns in rare cases, though modern purification practices significantly reduce this risk.
Another area where caution is advised involves individuals taking anticoagulant medications. Systemic proteolytic enzymes—those taken between meals for anti-inflammatory or fibrinolytic purposes—can influence blood viscosity or clotting dynamics. While digestive enzymes taken with meals do not typically exert systemic effects, high-dose protease supplements consumed on an empty stomach may increase the risk of bleeding in sensitive populations. For this reason, patients using warfarin, heparin, or other blood-thinning agents should consult a healthcare professional before incorporating proteolytic enzymes into their routine. This caution does not apply to most digestive enzymes used with food but is nonetheless important in the larger context of enzymatic supplementation.
Timing is one of the most critical determinants of enzyme effectiveness. When taken with the first bite of a meal—or within the first few minutes of eating—enzymes mix with the food bolus and begin working immediately as digestion progresses from the stomach to the small intestine. Taking enzymes too early may expose them to gastric acid without adequate food buffering, reducing their activity, while taking them too late may diminish their ability to participate in the earliest stages of nutrient breakdown. The exceptions to this timing rule include lactase, which is often taken immediately before consuming dairy, and systemic proteases, which are specifically intended to be taken on an empty stomach for non-digestive purposes.
Another key aspect of safe and effective supplementation involves understanding enzyme activity units. Unlike vitamins or minerals, whose dosages are measured in milligrams, enzymes are measured by their catalytic activity. This distinction is critical, because a high milligram amount does not necessarily indicate high potency; only validated activity units quantify how much substrate an enzyme can break down under standardized conditions. For example, amylase is measured in Dextrinizing Units (DU), protease in Hemoglobin Units Tyrosine base (HUT), lipase in Lipase Units (LU or FIP Units), lactase in Acid Lactase Units (ALU), and cellulase in Cellulase Units (CU). These activity-based measurements ensure that enzyme supplements deliver predictable digestive support and that manufacturing processes meet consistent standards.
Appropriate dosing varies depending on the individual’s digestive needs and the types of foods being consumed. People with mild digestive discomfort may respond well to modest multi-enzyme blends that support broad-spectrum digestion. Individuals with lactose intolerance often require lactase doses ranging from several thousand to nearly ten thousand ALU, depending on the dairy content of the meal. Those who struggle with high-fat meals may benefit from lipase-rich supplements formulated with several thousand LU, particularly if bile production or gallbladder function is compromised. Conversely, individuals with high protein intake or difficulty digesting meat may require higher protease activity, especially if stomach acid is insufficient to activate endogenous proteolytic pathways. In all cases, dosage is best adjusted gradually, allowing users to assess their response and modify intake based on meal size, composition, and symptom patterns.
Drug interactions, though uncommon, should be considered. Proton-pump inhibitors and antacids significantly alter gastric pH, which can reduce the activation of certain enzymes, especially those derived from animal sources. In such cases, fungal-derived enzymes—known for their broader pH tolerance—may offer more reliable activity. Antibiotics, while not directly interacting with enzymes, may temporarily disrupt the microbiome and alter the digestive environment; enzymes can help mitigate some of the gastrointestinal disturbances that accompany antibiotic therapy. Meanwhile, individuals using diabetes medications, particularly those affecting carbohydrate absorption, should be aware that certain carbohydrate-targeting enzymes may influence digestion rates slightly, though the effect is usually mild and clinically insignificant.
Digestive enzymes are safe for children, older adults, and individuals with chronic gastrointestinal conditions when used appropriately, though pediatric use should be supervised to ensure that symptoms are not masking underlying illnesses. Enzymes should not be used to justify the consumption of foods that provoke severe allergies, nor should they be relied upon to compensate for major dietary imbalances. Instead, they serve as a physiological complement—enhancing the body’s natural digestive capacity and reducing the burden on compromised digestive pathways.
Digestive enzyme supplementation remains one of the safest and most effective non-pharmaceutical interventions for enhancing digestive health. When guided by an understanding of timing, dosage, activity units, and individual physiology, enzyme therapy offers a predictable and well-tolerated means of improving digestive comfort and nutrient absorption. Its versatility across diverse digestive conditions further underscores the importance of thoughtful, evidence-based use in modern nutritional and clinical practice.
Supplement Formulation Science and the Stability Engineering of Digestive Enzymes
Formulating a high-quality digestive enzyme supplement is an intricate scientific and engineering challenge that extends far beyond merely combining protease, amylase, lipase, and other enzyme powders into a capsule. Unlike vitamins or minerals, digestive enzymes are three-dimensional protein structures whose catalytic function depends on the precise integrity of their folding pattern. This makes them innately sensitive to heat, moisture, oxidation, mechanical compression, and pH exposure. Achieving both stability and potency requires a sophisticated understanding of enzyme biochemistry, targeted delivery systems, specialized excipient selection, stringent environmental controls, and rigorous quality testing. In many respects, digestive enzyme formulation resembles pharmaceutical development more than traditional nutraceutical manufacturing.
The greatest threat to enzyme stability is moisture. Enzymes require a specific level of hydration to maintain their native conformation; too little hydration creates brittleness, while too much triggers partial unfolding, catalytic degradation, or even premature enzymatic activity within the capsule. For this reason, humidity control during manufacturing is not simply a procedural preference but an absolute necessity. Production workshops must operate at low relative humidity levels—often between 30% and 45%—to protect enzyme structure throughout blending, encapsulation, and packaging. Manufacturers equipped with dedicated low-humidity rooms, such as ZoomsHeal, are able to keep moisture-sensitive enzymes like protease, lactase, lipase, and cellulase stable during handling and ensure that their activity does not deteriorate before encapsulation. This level of environmental precision is essential for maintaining the enzyme’s intended therapeutic effect.
Heat poses a similar challenge. Enzymes denature when exposed to elevated temperatures, and even small fluctuations can irreversibly damage their catalytic sites. This is particularly relevant during tableting, where mechanical compression generates friction heat that can impair enzyme function. For this reason, capsules—especially those containing delayed-release or enteric-coated granules—are often preferred over tablets for enzyme delivery. Capsules avoid the thermal stress associated with tableting and allow formulators to preserve enzyme activity by minimizing heat and mechanical force. They also disintegrate more rapidly, which ensures timely release and interaction with food as it moves through the digestive tract.
The issue of pH stability further complicates formulation. Some enzymes, particularly those derived from animal pancreas, are extremely sensitive to acidic environments and lose activity rapidly in the stomach. To ensure that these enzymes reach the small intestine intact—where they are meant to function—enteric protection is required. Modern enteric systems employ pH-sensitive coatings such as HPMC derivatives, methacrylate polymers, and cellulose acetate phthalate. These coatings remain intact in the acidic pH of the stomach but dissolve at higher pH levels in the small intestine. Some formulations encapsulate enzymes into acid-resistant granules that are later filled into a standard capsule, providing flexibility and protecting enzymes without the need for fully enteric-coated capsules. Manufacturers must precisely calibrate the thickness, dissolution pH, and polymer ratio of enteric coatings to account for the enzyme’s acid sensitivity, the timing of release, and the intended digestive target. Facilities like ZoomsHeal rely on validated coating technologies and pH dissolution testing to guarantee that acid-sensitive enzymes are protected and delivered effectively into the small intestine.
Excipient selection also plays a crucial role in maintaining enzyme stability and ensuring uniform performance. Many commonly used tableting agents, binders, or lubricants interfere with enzyme activity, either by binding to the enzyme’s active site or by altering moisture dynamics within the formulation. Magnesium stearate, for example, is widely used in capsule and tablet manufacturing but may inhibit certain proteases if used excessively. Other excipients—particularly hygroscopic ones—can attract moisture into the formulation, posing risks for enzyme degradation. For this reason, formulators often rely on non-reactive fillers such as microcrystalline cellulose, rice flour, or calcium phosphate, and incorporate flow agents such as silica to maintain powder mobility without increasing moisture load. Excipient engineering becomes even more complex when formulating multi-enzyme blends, because each enzyme may respond differently to a shared excipient environment. Ensuring compatibility requires expertise, testing, and careful optimization.
The blending process itself must also be engineered with precision. Enzymes vary in particle size, density, and flow characteristics, making them prone to separation during mixing or transportation if not handled properly. Uniformity is critical: every capsule must deliver the exact activity specified on the label. This requires geometric dilution techniques, multi-stage blending, and gentle but thorough mixing to ensure an even distribution of enzyme particles throughout the excipient matrix. Manufacturers like ZoomsHeal implement controlled blending protocols and in-process sampling to verify that enzyme distribution remains consistent batch after batch.
Stability testing is another essential pillar of formulation science. Because enzymes naturally lose activity over time—even under ideal storage conditions—formulations must account for predictable degradation by incorporating appropriate overages. These overages are informed by accelerated stability studies conducted at elevated temperature and humidity, as well as real-time studies that track enzyme activity throughout shelf life. Every digestive enzyme product must undergo activity assays at multiple stages, including raw material testing, post-blending verification, finished-product analysis, and final testing near the end of its shelf life. Facilities with strong analytical capability, such as ZoomsHeal, perform FCC-standard activity assays for enzyme units such as DU (for amylase), HUT (for protease), LU or FIP (for lipase), CU (for cellulase), and ALU (for lactase). Ensuring consistent activity across the product’s lifespan is one of the most scientifically rigorous aspects of enzyme manufacturing.
Packaging engineering serves as the last line of defense for enzyme stability. Bottles must offer strong moisture and oxygen barriers, often accompanied by desiccant canisters or moisture-regulating sachets. Induction seals provide an airtight closure, and nitrogen flushing may be used to displace oxygen before final sealing. Blister packaging can offer additional stability but must be designed with high-barrier films to prevent moisture ingress. The packaging selected must match the enzyme’s sensitivity profile; lipase, for example, may require more stringent barriers than amylase or cellulase.
Digestive enzyme formulation is a sophisticated, multidisciplinary effort that blends biochemistry, material science, environmental control, and pharmaceutical-level quality assurance. The complexity of these requirements underscores why enzyme supplements vary so widely in effectiveness and why manufacturing expertise has become such a critical determinant of product performance. Facilities that possess controlled environments, advanced coating technologies, validated analytical methods, and experience handling sensitive biological actives—such as ZoomsHeal—are uniquely positioned to produce enzyme supplements that retain their potency from production through the final day of shelf life. As digestive enzymes continue to play a growing role in gastrointestinal health management, the importance of precise, scientifically grounded formulation will only become more pronounced.
ZoomsHeal as a Specialized Manufacturer of Digestive Enzyme Supplements
Manufacturing digestive enzyme supplements requires a level of technical sophistication that extends well beyond conventional nutraceutical production. The unique sensitivities of enzymes—particularly to heat, moisture, mechanical force, pH variations, and oxidative stress—demand facilities capable of maintaining strict environmental control, executing precise formulation engineering, and performing activity-specific analytical testing. In this highly specialized landscape, manufacturers who combine GMP-certified infrastructure with deep biochemical expertise occupy a critical role in ensuring that enzyme supplements deliver their intended physiological benefits. ZoomsHeal is one such manufacturer whose operational structure reflects these elevated technical requirements.
What distinguishes ZoomsHeal’s approach is not merely its compliance with GMP, ISO, and HACCP standards, but its focus on the nuanced scientific variables that govern enzyme stability. Digestive enzymes cannot be treated like ordinary powders; they are biologically active proteins whose functional lifespan is dictated by their exposure to moisture and temperature fluctuations during production. To address this, ZoomsHeal operates dedicated low-humidity manufacturing suites designed specifically for handling proteases, lipases, lactase, cellulases, and other moisture-sensitive enzymes. By maintaining relative humidity between 30% and 45%, these controlled environments prevent premature activation, hydrolysis, or structural degradation of enzymes during blending and encapsulation. This environmental precision is essential for ensuring that the catalytic activity specified on the product label remains intact through packaging and storage.
The manufacturing process further benefits from engineered workflows that minimize thermal stress and mechanical friction. Unlike facilities optimized primarily for tableting vitamins or minerals, ZoomsHeal employs encapsulation systems that reduce frictional heat and avoid the compression-based stress known to denature enzymes. When enteric delivery is required—particularly for pancreatin or acid-sensitive lipase—the company integrates pH-sensitive coating technologies that allow enzymes to bypass the stomach without loss of activity. These coating systems undergo dissolution testing to ensure that they release at the correct pH interval in the duodenum or jejunum. Such precision is necessary because even a slight deviation in coating thickness or polymer ratio can produce premature activation or impaired dissolution, compromising the supplement’s physiological effectiveness.
The formulation stage represents another area in which ZoomsHeal’s capabilities align closely with contemporary enzyme science. Because multi-enzyme blends include compounds with differing particle sizes, densities, and flow characteristics, they must be blended in a way that preserves uniformity. ZoomsHeal employs geometric dilution and multi-stage blending methodologies to ensure that each enzyme is evenly distributed throughout the excipient matrix. This is particularly important for high-potency formulations in which slight deviations in distribution could result in unequal dosing across capsules. Formulation scientists at ZoomsHeal also evaluate excipient compatibility using moisture-sorption profiles and interaction testing, selecting only those materials that maintain enzyme stability without binding to enzyme active sites or altering hydration levels.
Quality control represents perhaps the most technically demanding aspect of digestive enzyme production, as activity—not weight—determines the potency of the final product. ZoomsHeal utilizes FCC-standard enzymatic assays to quantify activities such as DU for amylase, HUT for protease, LU or FIP units for lipase, ALU for lactase, and CU for cellulase. Each batch undergoes multiple checkpoints, beginning with raw material verification, followed by in-process assays to confirm activity retention after blending, and concluding with final testing of the packaged product. These analytical capabilities are essential because enzyme degradation cannot be detected visually or through conventional physical inspections. Only activity assays provide a true indication of product performance.
Packaging engineering at ZoomsHeal reflects the same emphasis on biological stability. The company frequently employs nitrogen-flushed HDPE bottles, high-barrier induction seals, and pharmaceutical-grade desiccants to minimize oxygen and moisture exposure. When blister packaging is required, high-barrier composite films are used to protect enzymes from humidity fluctuations during transport and storage. These packaging choices are particularly important for lipase- and protease-heavy formulations, which are more susceptible to oxidative and moisture-driven denaturation.
Beyond technical manufacturing capacity, ZoomsHeal’s operational culture incorporates the iterative refinement characteristic of evidence-based formulation development. The company conducts ongoing stability studies, evaluating enzyme performance at accelerated aging conditions—such as 40°C and 75% relative humidity—to predict product behavior over its shelf life. Real-time stability monitoring further validates overage calculations and shelf-life claims, ensuring that enzyme activity remains within specification for the full duration of the product’s validity period. These practices align with international regulatory expectations for enzyme-containing supplements and reflect the company’s commitment to pharmaceutical-level quality standards.
In essence, ZoomsHeal’s role within the digestive enzyme sector is defined by its ability to translate complex biochemical requirements into stable, effective, commercially viable products. The interplay between enzyme structure, environmental conditions, excipient compatibility, and delivery technology demands a depth of understanding that extends far beyond standard supplement manufacturing. By operating facilities tailored for enzyme stability, employing validated analytical methodologies, and continuously refining its formulation strategies through scientific testing, ZoomsHeal provides a manufacturing platform capable of supporting both simple enzyme blends and advanced multi-enzyme complexes with precision.
As digestive enzymes continue to gain prominence in gastrointestinal health and personalized nutrition, the importance of technically competent manufacturing will only increase. Facilities like ZoomsHeal, which approach enzyme supplementation through the lens of biochemical engineering and rigorous quality science, will shape the next generation of digestive health products—products that are not only effective but also stable, consistent, and backed by a deep understanding of enzyme behavior within both biological and industrial systems.
The Evolving Role of Digestive Enzyme Supplements in Modern Health
Digestive enzyme supplements occupy a unique position at the intersection of nutrition science, clinical gastroenterology, and formulation engineering. What began decades ago as a supportive therapy primarily for individuals with severe pancreatic disorders has transformed into a wider field of research and application, driven by changing dietary patterns, shifts in population health, and a deeper understanding of digestive physiology. In today’s world—where diets have become more complex, lifestyles more stressful, and gastrointestinal conditions more prevalent—supporting the body’s digestive architecture through targeted enzyme supplementation is no longer a niche intervention but a rational response to widespread functional insufficiencies.
Throughout this article, we have explored the sophisticated biochemical choreography that underlies human digestion, from the earliest stages of salivary and gastric enzyme activity to the finely tuned processes occurring across the small intestine’s brush border. We have also examined the many pressures that erode enzymatic performance, whether they stem from aging, stress, inflammation, medication use, microbial imbalances, dietary extremes, or clinical diseases. In each case, insufficient enzymatic activity disrupts the flow of digestion, creating a cascade of effects that can compromise comfort, nutrient absorption, and intestinal equilibrium. Against this backdrop, supplemental digestive enzymes offer a targeted and evidence-supported means of restoring biochemical efficiency where the body struggles to keep pace.
The clinical evidence supporting digestive enzyme supplementation is both broad and compelling. Populations ranging from individuals with IBS, dyspepsia, lactose intolerance, or SIBO to those recovering from gallbladder removal or adapting to high-fat or high-protein diets have demonstrated meaningful improvements when exogenous enzymes are introduced. These improvements are grounded in mechanistic clarity: by completing digestive reactions that endogenous enzymes cannot, supplemental enzymes reduce fermentation, alleviate gastrointestinal stress, and optimize nutrient availability. Their benefits extend beyond symptomatic relief, influencing metabolic health, microbial balance, and overall digestive resilience.
Yet, the scientific promise of digestive enzymes can only be realized through careful and competent manufacturing. The delicate, protein-based nature of enzymatic structures requires an unusual level of precision during formulation, blending, coating, packaging, and storage. As we have discussed, enzymes are uniquely vulnerable to moisture, heat, pH shifts, and mechanical stress, making their stability dependent on advanced engineering and controlled environments. The work of manufacturers such as ZoomsHeal—who integrate low-humidity facilities, sophisticated blending protocols, pH-targeted coating technologies, and activity-based testing—demonstrates how the discipline of enzyme science must be paired with stringent industrial practices to create supplements that truly perform as intended.
It is within this context that digestive enzymes represent not merely a supplement category but an evolving scientific field. As research continues to unravel the complexities of digestive physiology and gut–brain interactions, enzyme formulations are likely to become increasingly specialized—tailored to individual microbiome profiles, dietary behaviors, genetic variations, and clinical needs. Innovations in encapsulation, time-release systems, enzyme engineering, and multi-target formulations will further enhance their ability to support human digestion in increasingly precise and personalized ways.
Ultimately, the value of digestive enzyme supplements lies in their alignment with a fundamental biological truth: digestion is a biochemical process that must function with remarkable precision for the body to thrive. When this precision falters—whether due to lifestyle, physiology, or disease—the consequences reverberate across the gastrointestinal system and beyond. Supplemental enzymes offer a direct, mechanistic intervention that reinforces the body’s natural digestive machinery, empowering individuals to maintain comfort, extract nutrients efficiently, and support metabolic and intestinal health in the face of modern dietary and environmental challenges.
As the demand for digestive health solutions continues to rise, the role of scientifically grounded enzyme supplementation will expand accordingly. The future of this field will be shaped not only by clinical insights but also by the expertise of manufacturers who understand that enzyme activity is both a biological and an engineering achievement. By combining rigorous science, responsible formulation, and advanced production capabilities, the next generation of digestive enzyme supplements will continue to elevate digestive wellness from a reactive approach to a proactive, evidence-based pillar of human health.